Video lecture for this page
This material (including images) is copyrighted!.
See my copyright notice for fair use
practices.
Hydrogen and helium and some lithium, boron, and beryllium were created when
the universe was created. All of the rest of the elements of the universe were
produced by the stars in nuclear fusion reactions. These reactions created the
heavier elements from fusing together lighter elements in the central regions
of the stars and through the explosion of white dwarfs or the merging of neutron stars. When the processed material from these processes are thrown back into space, it can be incorporated into gas clouds that will later form stars and planets.
The material that formed our solar system incorporated some of the remains of
previous stars.
All of the atoms on the Earth except hydrogen and most of the helium are
recycled material---they were not created on the Earth. They were created in
the stars.
The use of the word "created" here is different than what is normally meant
by scientists. In chemical reactions, different atoms or combinations of atoms
are said to be produced or created when a reaction takes place. For example,
in the Earth
section of the planets chapter,
I said that oxygen was produced in the photosynthesis
process of plants. That oxygen then goes into the air and you breathe it in. To
be more correct I should have said that the oxygen atoms
were moved or broken off from one set of compounds [carbon dioxide
(CO2) and water (H2O)]
to form a molecule of two oxygen atoms bound together (O2)
and a molecule of
carbohydrate made of carbon atoms, hydrogen atoms, and oxygen atoms
(C6H12O6). Each atom
is rearranged or re-used. It was much simpler to say that oxygen was
"created" as
a by-product of the photosynthesis process. I hope you did not mind. In defense
I want you to know that practically everyone, except for the astronomer
researching stellar evolution, uses this loose meaning of "creation" .
However,
now that you know about stellar nucleosynthesis, I need to be more careful
about what is being created from scratch and what is being re-used. Except for
the hydrogen and most of the helium atoms, all of the
materials around you, in the food you eat and drink, in the air you breathe,
in your muscles and bones,
in the paper and ink or toner of this book (or computer screen you are
reading), everything (!) are made of atoms that were created in the stars.
Those atoms are rearranged to produce the vast variety of things around and in
you. In the cores of stars, in supernova explosions, and merging neutron stars, new atoms are
manufactured from nuclear fusion reactions. You will find out where the
hydrogen and most of the helium atoms came from in the
cosmology chapter.
The atoms heavier than helium up to the iron and nickel atoms were made in the
cores of stars (the process that creates iron also creates a smaller amount
of nickel too). The lowest mass stars can only synthesize helium. Stars around
the
mass
of our Sun can synthesize helium, carbon, and oxygen. Massive stars
(M* > 8 solar masses) can
synthesize
helium, carbon, oxygen, neon, magnesium, silicon, sulfur, argon, calcium,
titanium, chromium, and iron (and nickel). Elements heavier than iron are made
in
supernova explosions from the rapid combination of the abundant neutrons with
heavy
nuclei as well as from the merger of neutron stars.
Massive red giants are also able to make small amounts of elements heavier than
iron (up to mercury and lead) through a slower combination of neutrons with heavy
nuclei but supernovae and merging neutron stars probably generate the majority of elements heavier than
iron and nickel (and certainly those heavier than lead up to uranium). The
synthesized elements are dispersed into the interstellar
medium during the planetary nebula or supernova stage (with supernova being the
best way to distribute the heavy elements far and wide). These elements will
be later incorporated into giant molecular clouds and eventually become part
of future stars and
planets (and life forms?)
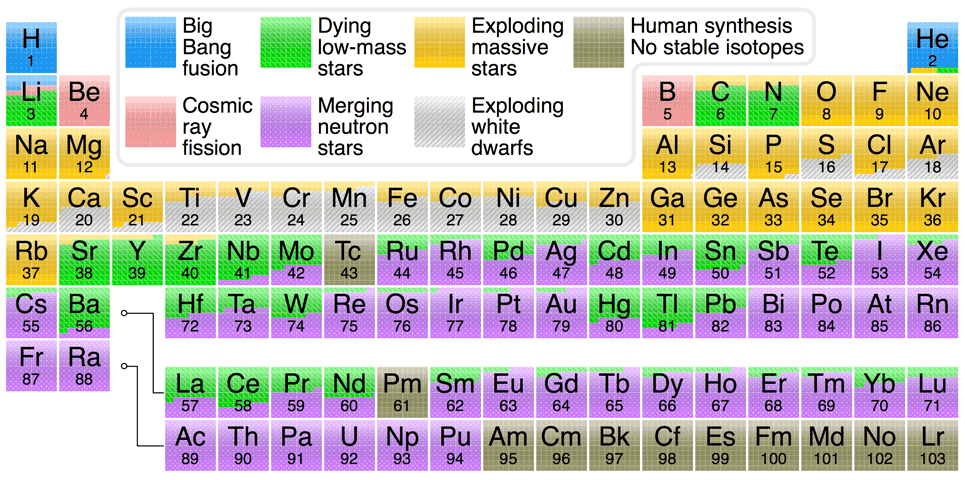
The periodic table chart at the bottom of this page summarizes the processes that have made the various elements in our solar system. Although the particulars of various nucleosynthesis
processes are beyond the scope of this website (see Johnson's "Origin of the Elements in the Solar System" blog entry and Ivans' "Origins of the Elements" website for the particulars), it is important to note a couple
of things:
-
The stellar nucleosynthesis theory correctly predicts the observed abundances
of all of the naturally occurring heavy elements seen on the Earth, meteorites,
Sun, other stars, interstellar clouds---everywhere in the universe. (In the
cosmology chapter you will see where the
hydrogen and most of the helium came from.) We understand now why some elements like carbon, oxygen, silicon, and iron are common and the heaviest elements like gold, mercury, and uranium are so rare.
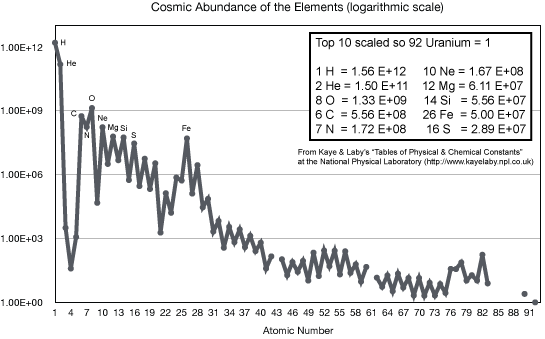
- In order to create a terrestrial planet like the Earth (and life on such a planet), enough of the heavy
elements have to be created in previous generations of stars and then concentrated
in the interstellar clouds to collect into sizable chunks around forming stars. There is necessarily a "lag"
between the beginning of the universe and the beginning of life.
 Go back to previous section --
Go back to previous section --
 Go to next section
Go to next section
last updated:
June 24, 2022
Is this page a copy of Strobel's
Astronomy Notes?
Author of original content:
Nick Strobel

