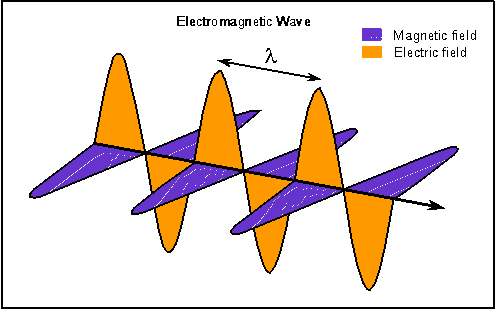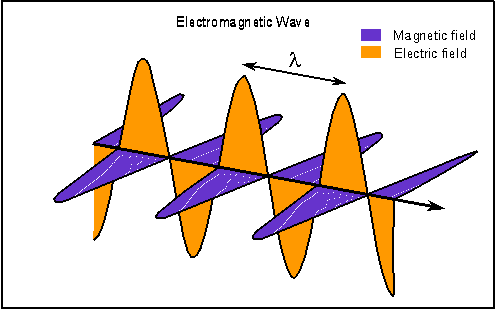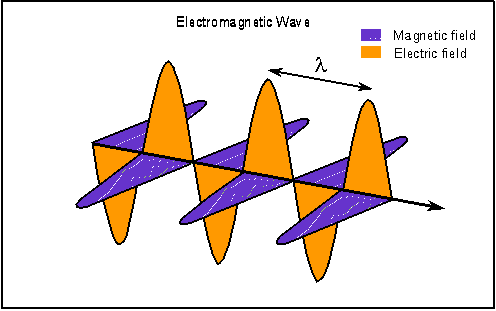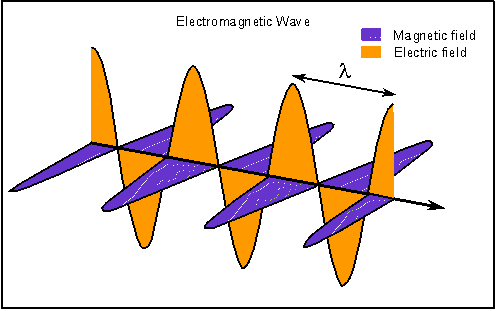
Electric and magnetic fields oscillate together but perpendicular to each other and the electromagnetic wave moves in a direction perpendicular to both of the fields.
Video lecture for this chapter

Light, electricity, and magnetism are manifestations of the same thing called electromagnetic radiation. The energy you see coming out of the computer screen you are using to read this page is made of fluctuating electric and magnetic energy fields. The electric and magnetic fields oscillate at right angles to each other and the combined wave moves in a direction perpendicular to both of the electric and magnetic field oscillations. This energy also comes in many forms that are not detectable with our eyes such as infrared (IR), radio, X-rays, ultraviolet (UV), and gamma rays.
We feel infrared light as heat and our radios pick up the messages encoded in radio waves emitted by radio stations. Ultraviolet light has high enough energy to damage our skin cells, so our bodies will produce a darker pigment in our skin to prevent exposure of the deeper skin cells to the UV (we tan as a defense mechanism). The special bulbs called ``black lights'' produce a lot of UV and were used by hospitals to kill bacteria, amoebas, and other micro-organisms. X-rays are produced by very hot things in space. X-rays have more energy than UV, so they can pass through skin, muscles, and organs. They are blocked by bones, so when the doctor takes your X-ray, the picture that results is the shadow image of the X-rays that passed through your body. Because X-rays have such high energy, they can damage or kill cells. A few brief exposures to low-intensity X-rays is okay. The X-ray technician would be exposed to thousands of X-ray exposures if s/he did not use some sort of shielding. Gamma rays are the most energetic form of electromagnetic radiation and are produced in nuclear reactions.
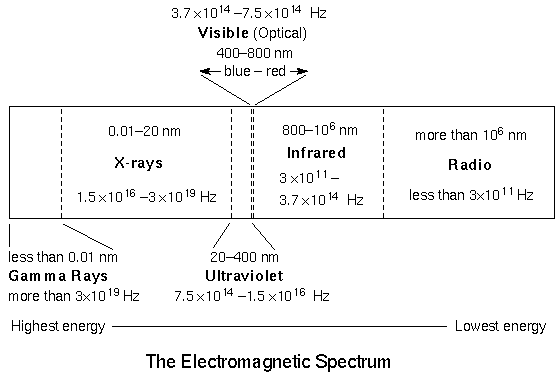
The form of electromagnetic radiation your eyes can detect is called ``visible'' or ``optical''. Astronomers have only recently (within the past few decades) been able to use the other forms of electromagnetic radiation or light. Every time technology has been developed to detect another form of light, a revolution in our understanding of the universe has occurred. The figure above shows all of the forms of electromagnetic radiation in order of INcreasing wavelength (given in nanometers (nm)) and DEcreasing energy. Notice how very narrow is the visible band! A beautiful poster describing the electromagnetic spectrum is available on the NASA SOFIA Public Outreach department's website (click the link to download the PDF of it or select the local link of the poster if the SOFIA link no longer works).
There are some general properties shared by all forms of electromagnetic radiation:
White light is made of different colors (wavelengths). When white light is passed through a prism or diffraction grating, it is spread out into all of its different colors. You see this happen every time you see a rainbow. In the prism, some colors in the white light travel slower through the prism than other colors, so the different colors are bent by different amounts to make the rainbow.

Not all wavelengths of light from space make it to the surface. Only long-wave UV, Visible, parts of the IR and radio bands make it to surface. More IR reaches elevations above 9,000 feet (2765 meters) elevation. That is one reason why modern observatories are built on top of very high mountains. Fortunately, as far as life is concerned, our atmosphere shields us from the gamma rays, X-rays, and most of the UV. It also blocks most of the IR and parts of the radio. Astronomers were not able to detect these forms of energy from celestial objects until the space age, when they could put satellite observatories in orbit.
Besides using wavelength to describe the form of light, you can also use
the
frequency--the number of crests of the wave that pass by a point
every
second. Frequency is measured in units of hertz (Hz): 1 hertz = 1
wave
crest/second. For light there is a simple relation between the speed of
light (c),
wavelength (![]() ), and
frequency (f):
), and
frequency (f):
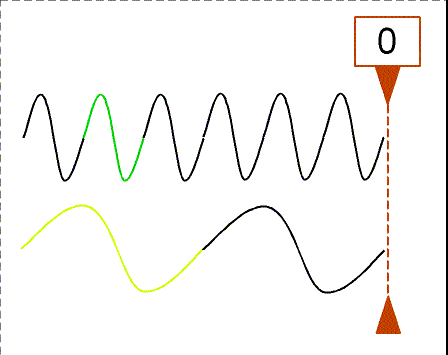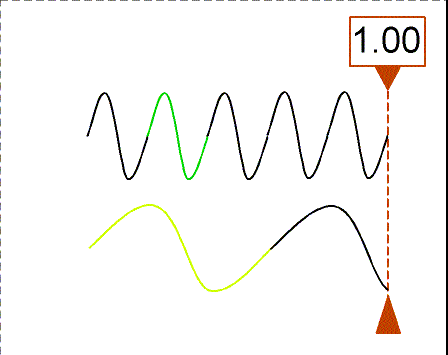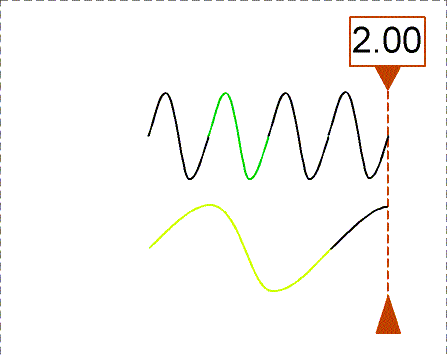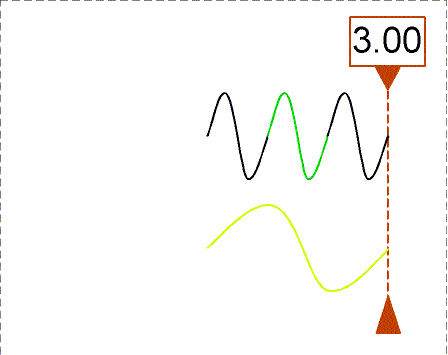
Some colors and their approximate wavelength, frequency and energy ranges are given in the table below. The unit of energy is the Joule (J). A Joule is how much energy you expend when you lift an object with 1 kilogram of mass (for example, a liter of water) about 10 centimeters above the ground. If you then let it go, the object hits the ground with that much energy. Sometimes light energy is also measured in ``ergs'', where 1 erg = 10-7 Joule.
| color | f (*1014 Hz) | Energy (*10-19 J) | |
|---|---|---|---|
| violet | 4000 |
7.5 |
5.0 |
| indigo | 4600 |
6.5 |
4.3 |
| blue | 4750 |
6.3 |
4.2 |
| green | 4900 |
6.1 |
4.1 |
| yellow | 5650 |
5.3 |
3.5 |
| orange | 5750 |
5.2 |
3.45 |
| red | 6000 |
5.0 |
3.3 |
 At the beginning of the 20th century Max Planck
(lived 1858--1947) suggested that atoms can absorb and emit energy in only
discrete chunks (called quanta). This quantum behavior of atoms
could explain the drop-off of a continuous
spectrum's shape at the short
wavelength end. A few years after Planck's discovery Albert
Einstein (lived 1879--1955) discovered that the quantum of energy was
not due to the atoms but, rather, a
property of the energy itself. You can consider
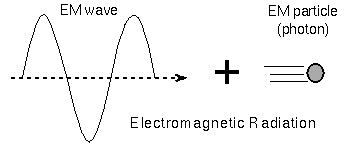
light as packets of energy called photons. A photon is a particle
of electromagnetic
radiation. Bizarre though it may be, light is both a particle and a wave.
Whether light behaves like a wave or like a particle depends
on how the light is observed (it depends on the experimental setup)!
At the beginning of the 20th century Max Planck
(lived 1858--1947) suggested that atoms can absorb and emit energy in only
discrete chunks (called quanta). This quantum behavior of atoms
could explain the drop-off of a continuous
spectrum's shape at the short
wavelength end. A few years after Planck's discovery Albert
Einstein (lived 1879--1955) discovered that the quantum of energy was
not due to the atoms but, rather, a
property of the energy itself. You can consider
light as packets of energy called photons. A photon is a particle
of electromagnetic
radiation. Bizarre though it may be, light is both a particle and a wave.
Whether light behaves like a wave or like a particle depends
on how the light is observed (it depends on the experimental setup)!
 Einstein found a very simple
relationship between the energy of a light wave (photon) and its frequency:
Einstein found a very simple
relationship between the energy of a light wave (photon) and its frequency:
| Energy of light | = | h × f |
| Energy of light | = | (h × c)/ |
Light can also behave as a particle and a wave at the same time. An example of light acting as both a particle and a wave is the digital camera---the lens refracts (bends and focusses) waves of light that hit a charge-coupled device (CCD). The photons kick electrons out of the silicon in the CCD. The electrons are detected by electronics that interpret the number of electrons released and their position of release from the silicon to create an image. Another example is when you observe the build-up of the alternating light and dark pattern from diffraction (a wave phenomenon) from light passing through a narrow slit. You see one bright spot (a photon), then another bright spot (another photon), then another... until the diffraction pattern is created from all of the accumulated photons. This happens so quickly that it is undetectable to the human eye.
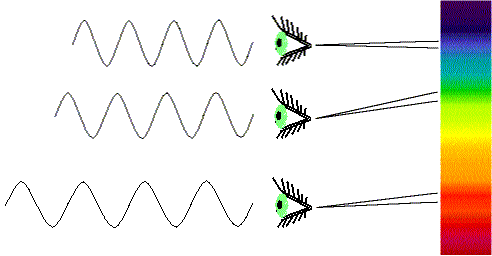
To decode the information stored in light, you pass the light through a prism or diffraction grating to create a spectrum---any display of the intensity of light (EM radiation) at different wavelengths or frequencies (a picture or a graph of intensity vs. either wavelength or frequency). If white light is examined, then the spectrum will be a rainbow.
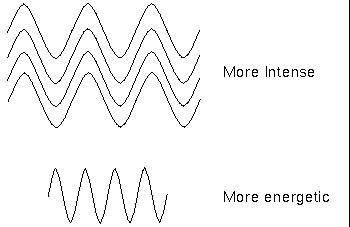
The term intensity has a particular meaning here: it is the number of waves or photons of light reaching your detector; a brighter object is more intense but not necessarily more energetic. Remember that a photon's energy depends on the wavelength (or frequency) only, not the intensity. The photons in a dim beam of X-ray light are much more energetic than the photons in an intense beam of infrared light.
The type of light produced by an object will depend on its temperature, so let's digress slightly to investigate what ``temperature'' is. Temperature is a measure of the random motion (or energy) of a group of particles. Higher temperature (T) means more random motion (or energy). A natural scale would have zero motion at zero degrees (absolute zero). This scale is the Kelvin scale. It scales exactly like the Celsius system, but it is offset by 273 degrees. Here is a comparison of the Kelvin, Celsius, and Fahrenheit temperature scales:
| K | C | F | |
|---|---|---|---|
| 0 | -273 | -459 | absolute zero |
| 100 | -173 | -279.4 | |
| 273 | 0 | 32 | water freezes |
| 310 | 37 | 98.6 | human temperature |
| 373 | 100 | 212 | water boils (STP) |
| 755 | 482 | 900 | oven on ``clean'' setting |
| 5840 | 5567 | 10053 | Sun's temperature |
| electromagnetic radiation | frequency | hertz |
|---|---|---|
| intensity | Kelvin | photon |
| spectroscopy | spectrum | temperature |
| wavelength |
![]() Go back to previous section --
Go back to previous section --
![]() Go to next section
Go to next section
last updated: January 13, 2022