Video lecture for this chapter
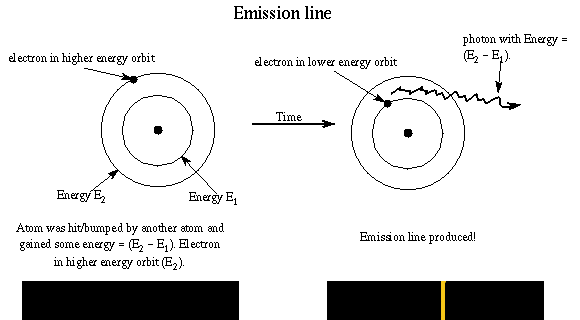
Let's see how Bohr's model of the atom explains the three types of spectra. An emission line is produced by an atom in a ``excited'' energy state---the electron is not in as low an energy orbit as possible. Remember rule #3! In order to go to a lower energy orbit, the electron must lose energy of a certain specific amount. The atom releases the energy is the form of a photon with that particular energy. The energy of photon = the difference in energy of the energy orbits (energy ladder rungs).
Example: An atom with an electron at the E2 orbit and wants to get to the lower E1 energy orbit. It gives off a photon with energy E = h × f = E2 - E1. The electron may reach the ground state in one jump or it may temporarily stop at one or more energy levels on the way, but it canNOT stop somewhere between the energy levels. Different jumps produce photons of different energies. A larger jump to a lower energy level, will produce a photon with greater energy (smaller wavelength).
The atom produces light of certain wavelengths. (Remember that light is both a photon and a wave!) The more atoms undergoing a particular transition, the more intense the emission line will be. The intensity depends on the density and temperature of the gas.

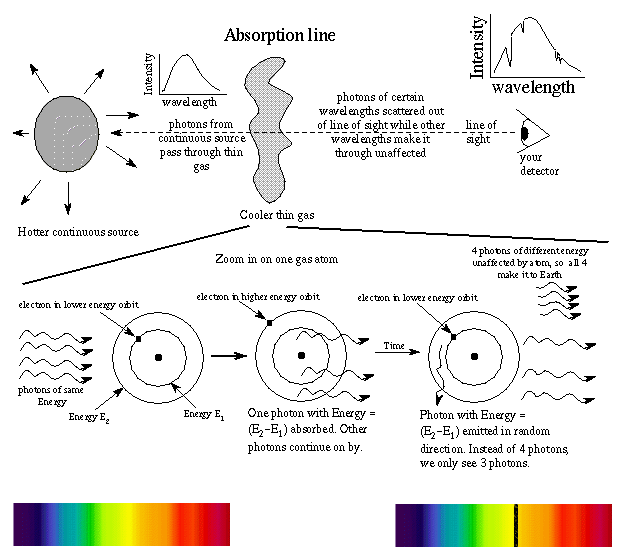
An absorption line is produced when a photon of just the right energy is absorbed by an atom, kicking an electron to a higher energy orbit. The photon had energy = the difference in energy of the energy orbits. Because the energy levels in an element's atoms are fixed, the size of the outward jumps made by the electrons are the same as the inward jumps. Therefore, the pattern of absorption lines is the same as the pattern of emission lines. Other photons moving through the gas with the wrong energy will pass right on by the atoms in the thin gas. They make up the rest of the continous spectrum you see.
Example: An atom with electron in the E1 orbit sees a photon with energy Ephoton = E2 - E1. The photon is absorbed and electron moves to E2. The photon is later re-emitted but in a random direction---not necessarily in the same direction as the original photon! An observer will see less photons from the direction of the continuous source at that specific frequency (color) than other frequencies (colors). Photons of other energies pass right on by without being absorbed. The atom can absorb photons of just the right energy to move an electron from one energy level to another level. (See the "How do you do that?" box below for a more detailed example of the absorption lines for hydrogen.) The more atoms undergoing a particular absorption transition, the darker (or ``stronger'') the absorption line. The strength of the absorption line depends on the density and temperature.
How do you do that?Find the energies of the photons that can be absorbed by the hydrogen atom if the hydrogen gas is hot enough for its electron to usually be in the second energy level. (We'll see in chapter 11 that this is the case for A and B-type stars.) In this example, we will use the standard physics convention and have the values of the energy levels be negative numbers to mean that the electrons in the levels are bound to the atom. In this convention, an electron with positive energy is free of the atom (unbound). Also, we will use the energy unit called “electron volt” (eV), where 1 eV = 1.602 x 10-19 Joules to make the numbers easier to work with. Only four of the many energy levels are shown in the figure below. The higher energy levels get too crowded together to distinguish them in the picture but here are the next two higher levels: n=5 at Energy E = -0.54 eV and n=6 at E = -0.38 eV. Energy hops possible are:
The spectrum shows the hydrogen absorption line energies. The line at 2.86 eV is for the n=2 to n=5 hop and the line at 3.02 eV is for the n=2 to n=6 hop. Photons with energies between 1.89 and 3.4 eV not listed above will NOT be absorbed by the atom. |
A thermal spectrum is produced by atoms that are closely packed together. The energy levels of the atoms are distorted by their neighboring atom's electrons. This smears out the normally sharp spectral lines (they become fatter).
Example: An orange line is fattened so that one edge is in the yellow wavelengths and the other edge is in the red wavelengths. The amount of smearing, or broadening, depends on the density. Eventually, the density gets high enough to where the smeared lines all merge together to produce the rainbow of colors of a continuous spectrum.
Use the UNL Astronomy Education program's Hydrogen Energy Levels module to further explore how emission and absorption lines are created (link will appear in a new window). You can also see how the number of atoms in a given state (electrons in a given energy level) changes with the temperature.
The pattern of spectral lines and particular wavelengths produced by an atom depend very sensitively on the masses and charges of the sub-atomic particles and the interactions between them (forces and rules they follow). If different parts of the universe had even slight differences in the rules of quantum mechanics that govern the interactions of the protons, electrons, and neutrons or differences in the strengths of the fundamental forces of nature from that observed on the Earth, we would see noticable changes in the spacing and strength of the spectral lines. If the subatomic particles had different amount of charge or mass, the pattern of lines would be different than what you see on the Earth. We observe that all hydrogen atoms produce the same pattern of lines everywhere in the universe, whether they are in the Sun, other stars, distant galaxies, or intergalactic space. (Hydrogen is not special in this regard---all of the other types of atoms produce the same pattern that is unique to that type of atom.) The fact that we see the same pattern of lines for a given element (type of atom) everywhere in the universe tells us that the same laws of physics used in the structure of atoms work everywhere in the universe.
Recall from the first chapter that astronomy gives us a sort of time machine: when we look at very distant objects we see them as they were a long time ago because light, traveling at a fast but finite speed, has taken a long time to travel the vast distances between us and the distant objects. The light from those distant objects tells us about the physical laws operating in that part of the universe when the light was emitted long ago. The fact that we see the same pattern of lines for a given element in light emitted by objects at any time in the universe tells us that the laws of physics are the same throughout time---they do not change.
You will also see in the following chapters that the same kind of conclusions can be reached by looking at the gravitational interactions of objects at any position and distance from us. We see the same laws of nature operating at all places and times in the universe. If you reflect a bit on that fact, it is pretty darn amazing!
![]() Go back to previous section --
Go back to previous section --
![]() Go to next section
Go to next section
last updated: January 13, 2022