Video lecture for this chapter
This material (including images) is copyrighted!.
See my copyright notice for fair use
practices. Select the photographs to display the original source in
another window.
Scientists have had the technology to observe discrete spectra since the
beginning of the 19th century. They had to wait over a hundred years, though, for
an explanation of how the discrete spectra were produced. They knew that it was
produced by atoms and that atoms had negative and positive charges in them.
Some models of the atom were similar to our current one: the positive charges
are concentrated in a central nucleus with the negative charges swarming around
it, but the atoms should be unstable. As the negative charges (called
electrons) move around the nucleus, they should radiate light and spiral
into the nucleus in about 10-16 second. This is obviously contradicted by
common experience!
 Niels Bohr (lived 1885--1962) provided the explanation in the early 20th
century. He said that the electron can be only found in energy orbits of a
certain size and as long as the electron is in one of those special orbits, it
would radiate no energy. If the electron changed orbits, it would radiate or
absorb energy. This model sounds outlandish, but numerous experiments have
shown it to be true.
Niels Bohr (lived 1885--1962) provided the explanation in the early 20th
century. He said that the electron can be only found in energy orbits of a
certain size and as long as the electron is in one of those special orbits, it
would radiate no energy. If the electron changed orbits, it would radiate or
absorb energy. This model sounds outlandish, but numerous experiments have
shown it to be true.
In Bohr's model of the atom, the massive but small positively-charged
protons and massive but small neutral neutrons are
found in the tiny nucleus. The small, light negatively-charged
electrons
move around the nucleus in certain specific orbits (energies).
In a neutral atom the number of electrons = the number of protons.
The arrangement of an atom's energy orbits depends on the number of protons
and neutrons in the nucleus and the number of electrons orbiting the nucleus.
Because every type of atom has a unique arrangement of the energy orbits, they
produce a unique pattern of absorption or emission lines.
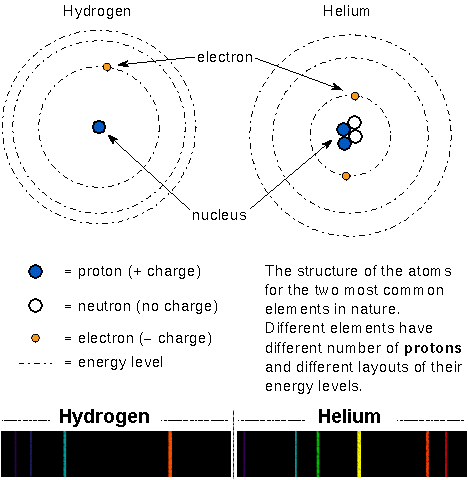
Select here for an enlargement of the spectra.
All atoms with the same number of protons in the nucleus are grouped together
into something called an element. Because the atoms of an element have
the same number of protons, they also have the same number of electrons and,
therefore, the same chemical properties. For example, all atoms with one
proton in the nucleus have the same chemical properties and are called
Hydrogen. All atoms with two protons in the nucleus will not chemically react
with any other atoms and are known as Helium. The atoms called Carbon form the
basis of life and have six protons in the nucleus.
In the figure below, atom (a) is Hydrogen, atom (b) is Helium,
atoms (c), (d), and (e) are Lithium.
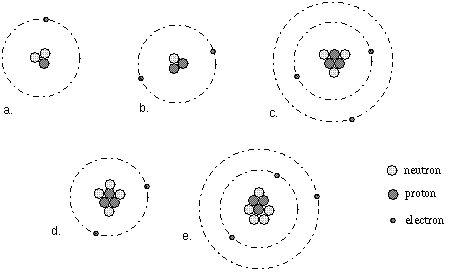
Elements are sub-divided into sub-groups called isotopes based on the
number of protons AND neutrons in the nucleus. All atoms of an element with the
same number of neutrons in the nucleus are of the same type of
isotope.
An element's isotopes will have very nearly the same chemical properties but
they can behave very differently in nuclear reactions. For example,
all of the isotopes of the element Hydrogen have one electron orbiting the
nucleus and behave the same way in chemistry reactions.
The ordinary Hydrogen isotope has 0 neutrons + 1 proton while another
Hydrogen isotope called Deuterium has 1 neutron + 1 proton and another
Hydrogen isotope called Tritium has 2 neutrons + 1 proton in the
nucleus. Tritium is radioactive---its nucleus spontaneouly changes into
another
type of nucleus. In the figure above, atoms (c), (d), and (e) are different
isotopes of the same element called Lithium.
Most atoms in nature are neutral, the negative charges exactly cancel the
positive charges. But sometimes an atom has a hard collision with another atom
or absorbs an energetic photon so that one or more electrons are knocked out
of the atom. In some rare cases, an atom may temporarily hold onto an extra
electron. In either case, the atom has an extra positive or negative charge and
is called an ion. For example, the carbon ion C+ has
6 protons and 5 electrons and the iron ion Fe2+ has 26
protons and 24 electrons. Because the number of electrons are different, an ion
of an element will behave differently in chemical reactions than its neutral
cousins. In the figure above atom (d) is a Li+ ion [compare
it with atom (c) or (e)].
In order to explain discrete spectra, Bohr found that atoms obey three
basic rules:
- Electrons have only certain energies corresponding to particular
distances from nucleus. As long as the electron is in one of those energy
orbits, it will not lose or absorb any energy. The energy orbits are analogous
to rungs on a ladder: electrons can be only on rungs of the ladder and not in
between rungs.
- The orbits closer to the nucleus have lower energy.
- Atoms want to be in the lowest possible energy state called the
ground state (all electrons as close to the nucleus as possible).
 Go back to previous section --
Go back to previous section --
 Go to next section
Go to next section
last updated:
January 13, 2022
Is this page a copy of Strobel's
Astronomy Notes?
Author of original content:
Nick Strobel
 Niels Bohr (lived 1885--1962) provided the explanation in the early 20th
century. He said that the electron can be only found in energy orbits of a
certain size and as long as the electron is in one of those special orbits, it
would radiate no energy. If the electron changed orbits, it would radiate or
absorb energy. This model sounds outlandish, but numerous experiments have
shown it to be true.
Niels Bohr (lived 1885--1962) provided the explanation in the early 20th
century. He said that the electron can be only found in energy orbits of a
certain size and as long as the electron is in one of those special orbits, it
would radiate no energy. If the electron changed orbits, it would radiate or
absorb energy. This model sounds outlandish, but numerous experiments have
shown it to be true.

